By decision of the United Nations, we are in the International Year of the Periodic Table, celebrating the 150th anniversary of its proposal by Russian chemist Dmitri Mendeleiev.
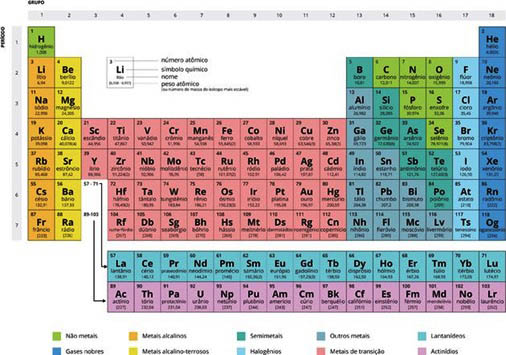
That table summarizes, on a simple A4 sheet, all 118 chemical elements known to date, arranging them according to their properties.
All known matter – the Universe, the Solar System, the Earth, the human body – is made of these 118 elements, naturally in very different abundances. They are like Lego pieces for the cosmic construction game. And we only need a box with 118 different parts to obtain, connecting them, the infinite possibilities of the chemical world.
The elements of the same column of the Periodic Table are similar because the atoms that constitute them are similar, but, in Mendeleev's time, there was not even the certainty of the existence of atoms, and their structure was completely unknown: neither was known nor the atomic nucleus, nor the electrons that circulate around it.
We now know from quantum theory, established only at the beginning of the twentieth century, that occupations by electrons of quantum states of the same type explain the similarities in the chemical behavior of atoms.
Some atoms bond easily, such as those in the first column (so-called alkali metals), while those in the last column bond with difficulty (called rare gases).
The unifying vision of the creator of the Table was extraordinary, as was the vision of the English naturalist Charles Darwin when he discovered in 1859 a universal order in the extreme diversity of the living world, which modern genetics only later came to explain.
The Universe is essentially made up of the two lighter chemical elements, which occupy the first two horizontal spaces of the Periodic Table and which also head the first and last columns: hydrogen (which makes up 75% of the total mass of matter in the Universe) and helium (which makes up 23% of the total mass).
Both were formed at the Big Bang 14 billion years ago, but some of today's helium was produced in stars from hydrogen.
In fact, stars like the Sun are nothing more than nuclear power plants in which hydrogen is permanently transformed into helium.
Across the Universe, the elements that appear next are much less abundant: soon after comes oxygen (1%), which is formed in certain stars that make up the galaxies, spread throughout the Universe.
The Sun will be able to make carbon at the end of its life from helium, but larger stars are already needed to make significant amounts of oxygen.
Hydrogen and helium were discovered at very different dates and by very different means. Hydrogen was identified by the English physicist and chemist Henry Cavendish in 1766, studying chemical reactions of acids with metals, whereas helium only appeared in 1868, when astronomers Jules Jansen, French, and Norman Lockyer, English, analyzed the light of Sol (helium means Sun in Greek). It was the first and only element to be discovered outside of Earth.
Oxygen is contemporary with the emergence of Chemistry, discovered in 1772-1774 by chemists Carl Scheele, Swedish, and Joseph Priestley, English (the French chemist Antoine-Laurent Lavoisier, also claimed the discovery of oxygen).
On Earth, the abundance of elements is very different from that which occurs in the Universe in general, which is practically the same as in the solar system (whose mass is dominated by that of the Sun).
The most abundant elements of the earth's crust are, in percentage by mass, oxygen (46%), silicon 28% and aluminum (8%).
As with oxygen, silicon and aluminum can only be made into heavy stars. All three were scattered in space by supernovae, which are huge explosions of massive stars that occur when they reach the end of their lifetime.
This means that there was a star before the Sun that exploded violently, scattering its matter into space. The Sun is therefore a second generation star.
Like helium, silicon and aluminum were identified in the 1824th century, and almost at the same time: silicon was discovered in 1825 by the Swedish chemist Jöns Jacob Berzelius and aluminum in XNUMX by the Danish physicist Hans-Christian Oersted.
Finally the man. Most of the human body is water (H2O), the rest being dominated by organic molecules, which contain carbon.
As a percentage of mass, in the human body it is formed mostly by oxygen (65%), followed by carbon (19%) and hydrogen (10%). The rest (nitrogen, calcium, potassium, etc.) is just a few tiny crumbs.
Of the 118 elements in the Table, only 17 are necessary for our body to function. The most extraordinary thing is that, with the exception of hydrogen, which came from the Big Bang, all 17 of these came from the stars: we are, therefore, “children” of the stars.
Not knowing we other intelligent living beings, we are the only “children” of the stars who can perceive where they came from.
Author Carlos Fiolhais (Professor of Physics at the University of Coimbra)



















Comments