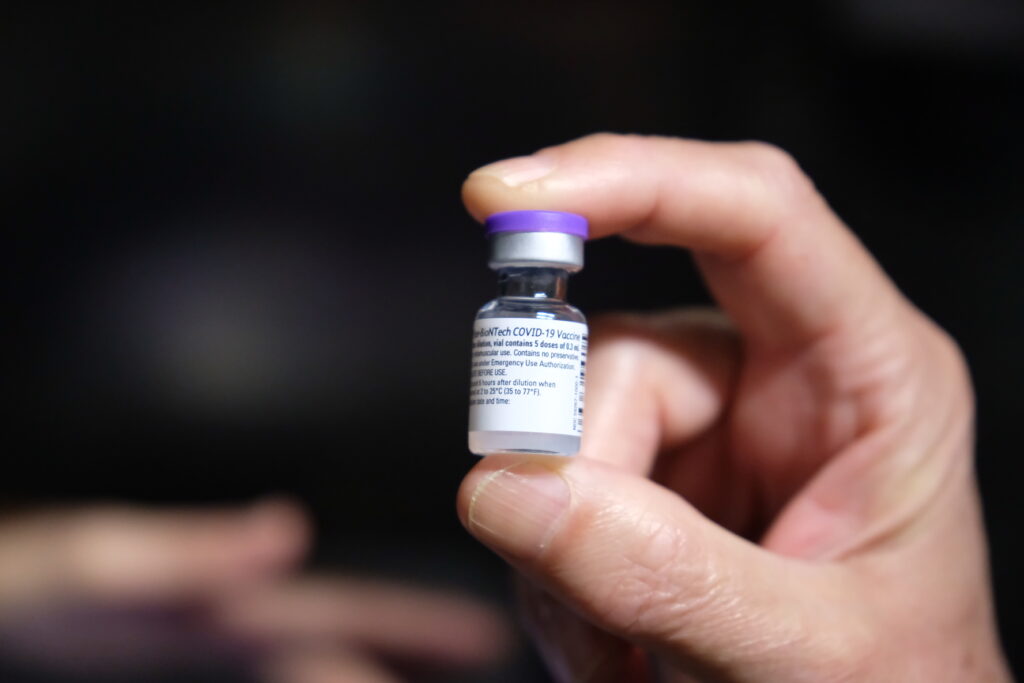
The European Medicines Agency (EMA) approved this Thursday, November 25, the administration of the vaccine against covid-19 by BioNTech/Pfizer, to children from 5 to 11 years old, being the first in the European Union (EU) for this age group.
"The EMA Committee for Medicinal Products for Human Use recommended granting an indication extension for the Comirnaty vaccine [brand name for the vaccine from the BioNTech/Pfizer pharmaceutical consortium] to include use in children aged 5-11 years," he says. the European regulator in a statement.
The vaccine was already used from the age of 12 onwards.
The EMA explains that for children aged 5 to 11 years, the dose of Comirnaty "will be less than that used in people aged 12 and over", but "as in the older age group, it is given as two injections in forearm muscles, three weeks apart».
This is the first vaccine approved in the EU for children in this age group, at a time when there is an increase in cases at these ages and when the United States is already administering it.


















Comments